

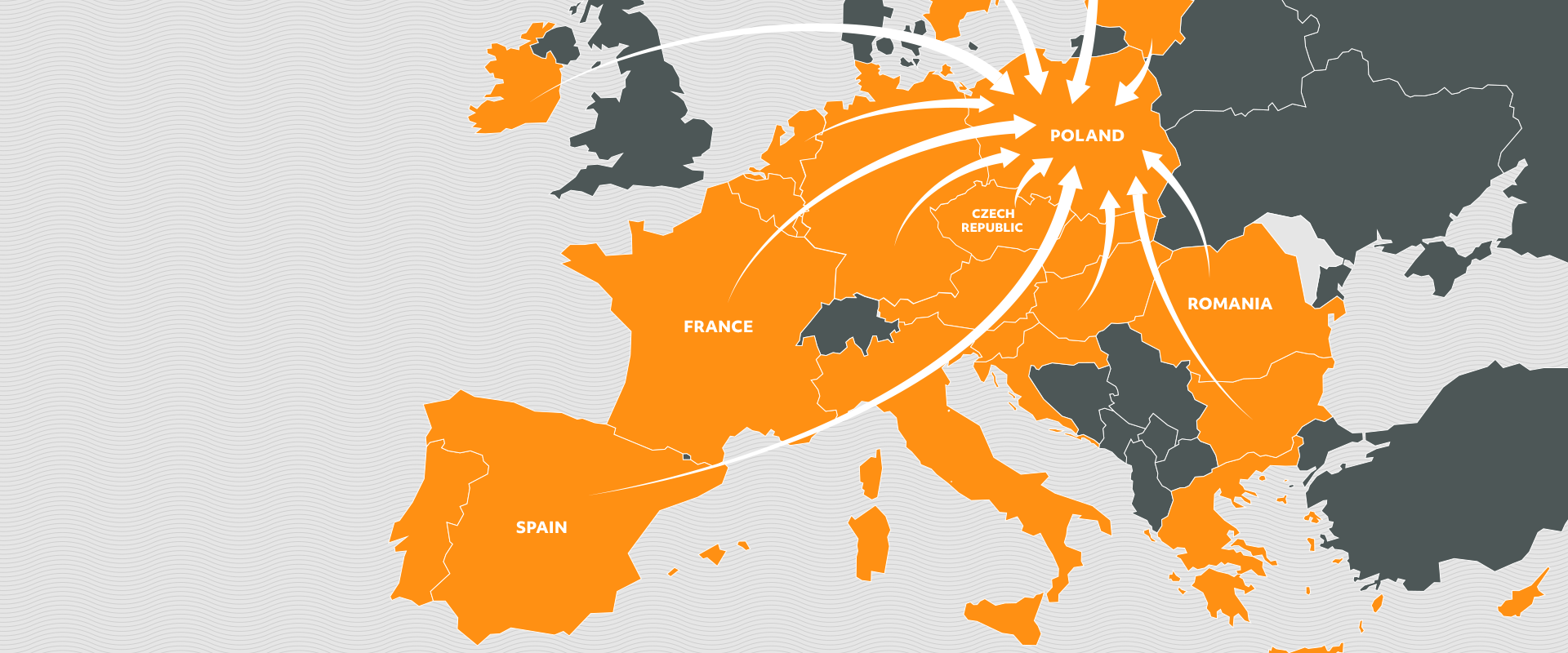
It is a type of turnover of goods between the countries of the European Economic Area which consists in purchasing the medicines in the cheapest market possible, repacking them into new packages and providing them with the translated leaflets. Then the medicines are distributed to the pharmaceutical warehouses and pharmacies in prices lower than the lowest local price.
All stages of the process of parallel import and distribution of medicines are specialty supervised by competent bodies: Te Main Pharmaceutical Inspector, Minister of Health, and the President of the Office for Registration of Medicinal Products for Medical Devices and Biocidal Products.
Medical product from the parallel import contains, inter alia, the same active substance, same power and method of administration as the medical product with the permit for turnover in Poland. One shall not mistake it for the equivalents, meaning the generic drugs.
Products coming from the parallel import are even several dozen of percent cheaper than the medicines distributed in the traditional way.
Issuing a permit that each medicine that comes from the parallel import hold a permit issued by the Office for Registration of Medicinal Products for Medical Devices and Biocidal Products Repacking.
The medical products are repacked into new Polish language version packages. Next, the medicines undergo serialization according to the requirements of the Anti-counterfeiting Directive (EU FMD) since 9 Feb., 2019r.
The medicines are stored and distributed under controlled conditions into the pharmaceutical warehouses and pharmacies.

 License number
License number The package of the parallel import medicine
can slightly differ from the referring local
product. However, it is the same product
yet with a lower price. It contains i.e.
the same active substances, power
and the method of administration.
One shall not mistake it for the generic
drugs i.e. the equivalents.